Hello Students we are providing mcqs of class 12 Chemistry lesson 7 P-Block Elements These objective questions is very helpful for your study because these question cover a wide topic and easy to understand
Why mcqs of class 12 Chemistry lesson 7 P-Block Elements is important ?
- these mcqs are important because these questions cover a wide topic of the chapter
- mcqs are easy to understand
- mcqs break the big topic in some small topic so they are easy to learn
- they give a short revision at the time of class 12 Chemistry exam
How to get pdf of class 12 Chemistry lesson 7 P-Block Elements mcqs
We are providing 3 types of format from where you can learn and download the mcqs of class 12 Chemistry P-Block Elements
1. You can watch our video on Youtube
2. You can get mcqs at our website
3. you can download pdf of mcqs
We have provided objective questions of class 12 Chemistry lesson 7 P-Block Elements with answers these questions help to understand the concept very easy for student
Increasing role of mcqs in board exam
recently many examination board have declared that their question paper of exam contain more than 25% objective questions so by the marks purpose mcqs of class 12 Chemistry lesson 7 P-Block Elements is very important
We hope that the provided mcqs of class 12 Chemistry lesson 7 P-Block Elements are very helpful for your study and you get full marks in class 12 Chemistry exam if you have any queries about objective questions of class 12 Chemistry chapter 7 P-Block Elements you can comment below the post or you can go to our contact us page
Elements having +5 oxidation state is
A. Bismuth
B. Phosphorous
C. Arsenic
D. Antimony
Answer - B
Explanation - The maximum oxidation state shown by a p-block element is equal to the total number of valence electrons (i.e., the sum of the s and p-electrons).
P :[Ne]3s23p3. Total valence electrons = 5 so it will show +5 oxidation state. The stability of +5 oxidation state decreases down the group due to the inert pair effect.
[Ar]3d104s24p3 is the electronic configuration of
A. Antimony
B. Bismuth
C. Arsenic
D. Phosphorous
Answer - C
Explanation - Arsenic is a group 15 element and its atomic no. is 33.
The most common oxidation states of nitrogen are
A. +3, +5
B. – 3, +5
C. – 3, +3
D. – 3, +3, +5
Answer - D
Explanation - N shows -3 in NH3, +3 in HNO2 and +5 in HNO3.
In which of the following compound, nitrogen shows the oxidation state of +5?
A. N2
B. HNO3
C. NH3
D. NH2OH
Answer - B
Explanation - Oxidation state of O is -2 and H is +1. Let the oxidation state of N is x.
(+1) +x + 3(-2) = 0
x = +5
The maximum covalency of nitrogen is
A. Four
B. Six
C. Eight
D. Five
Answer - A
Explanation - N has maximum covalency of 4 since only four (one s and three p) orbitals are available for bonding. d orbitals are not available for bonding.
Which among the following forms basic oxide?
A. Antimony
B. Nitrogen
C. Bismuth
D. Phosphorous
Answer - C
Explanation - As we move down the group in the periodic table, the metallic character increases so Bi is a metal thus its oxide is basic.
In laboratory ammonia is prepared by heating
A. Nitrogen and hydrogen
B. Ammonium chloride with calcium hydroxide
C. Calcium cyanamide with water
D. Ammonium chloride with sodium hydroxide
Answer - D
Explanation - On a small scale ammonia can be prepared by treating ammonium chloride or ammonium sulphate with sodium hydroxide or calcium hydroxide. It is also prepared by hydrolysis of magnesium nitride.
Ammonia has a higher boiling point and is less volatile because of
A. Dipole – Dipole interaction
B. Covalent bond
C. Hydrogen bond
D. Vander waal forces
Answer - C
Explanation - In the solid and liquid states NH3 is associated through intermolecular hydrogen bonding and therefore it has higher boiling point and less volatile compared to other hydrides of group 15.
Which compound is used in the preparation of caprolactam?
A. Phosphine
B. Ammonia
C. Nitric acid
D. Phosphorous halide
Answer - B
Explanation - NH3 is used in the preparation of caprolactam. Caprolactam is an organic compound with the formula (CH2)5C(O)NH. It is used to make Nylon 6 fibre and plastics.
Which compound is used as the cooling liquid in refrigerators?
A. Ozone
B. Ammonia
C. Phosphine
D. Nitrous oxide
Answer - B
Explanation - Liquid NH3 is used as a refrigerant. evaporation of a liquid needs heat energy. when liquid ammonia vapourises, it absorbs large quantities of heat without changing its temperature.
Ammonia act as a Lewis base because nitrogen has
A. It has pyramidal structure
B. 3 electrons in the outermost shell
C. Bond pair of electrons
D. Lone pair of electrons
Answer - D
Explanation - Lewis base is a substance which can donate lone pair of electrons. NH3 has one lone pair of electrons on the nitrogen atom therefore can act as Lewis base.
Which allotrope of phosphorous is most stable?
A. Blue
B. Black
C. White
D. Red
Answer - B
Explanation - Black Phosphorus is the most stable isotope of phosphorus because it is a highly polymerized form of phosphorous.
At what temperature white phosphorous changes to red phosphorous?
A. 373 K
B. 673 K
C. 573 K
D. 473 K
Answer - C
Explanation - White phosphorus when heated to 573 K in an inert atmosphere for several days red Phosphorus is obtained.
What is the spontaneous ignition temperature of white phosphorous?
A. 20°C
B. 15°C
C. 10°C
D. 35°C
Answer - D
Explanation - The spontaneous ignition temperature of white phosphorus is 35°C. it is due to angular strain in the P4 molecules where the angles are only 60°.
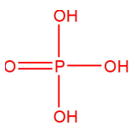
is the structure of:-
A. Phosphinic acid
B. Phosphorous acid
C. Orthohosphoric acid
D. Pyrophosphoric acid
Answer - C
Explanation - This structure is of H3PO4. (Orthophosphoric acid). It has three P-OH and one P=O bond.
mcqs for cbse term 1 exam
mcqs for cbse term 1 exam class 12
mcqs for cbse term 1 exam class 12 Chemistry
mcqs for cbse term 1 exam class 12 Chemistry lesson 7
mcqs for cbse term 1 exam class 12 Chemistry lesson 7 P-Block Elements
mcqs for cbse term 1 exam class 12 Chemistry chapter 7
mcqs for cbse term 1 exam class 12 Chemistry chapter 7 P-Block Elements
MCQ'S TEST
mcqs test
class 12
class 12 Chemistry
class 12 Chemistry chapter 7
class 12 Chemistry chapter 7 P-Block Elements
class 12 Chemistry lesson 7
class 12 Chemistry lesson 7 P-Block Elements
class 12 mcqs
class 12 Chemistry mcqs
class 12 Chemistry chapter 7 mcqs
class 12 Chemistry chapter 7 P-Block Elements mcqs
class 12 objective questions
class 12 Chemistry objective questions
class 12 Chemistry chapter 7 objective questions
class 12 Chemistry chapter 7 P-Block Elements objective questions
mcqs of class 12
mcqs of class 12 Chemistry
mcqs of class 12 Chemistry chapter 7
mcqs of class 12 Chemistry chapter 7 P-Block Elements
objective questions of class 12
objective questions of class 12 Chemistry
objective questions of class 12 Chemistry chapter 7
objective questions of class 12 Chemistry chapter 7 P-Block Elements
class 12 Chemistry lesson 7
class 12 Chemistry lesson 7 P-Block Elements
class 12 Chemistry lesson 7 mcqs
class 12 Chemistry lesson 7 P-Block Elements mcqs
class 12 Chemistry lesson 7 objective questions
class 12 Chemistry lesson 7 P-Block Elements mcqs objective questions
mcqs of class 12 Chemistry lesson 7
mcqs of class 12 Chemistry lesson 7 P-Block Elements
objective questions of class 12 Chemistry lesson 7
objective questions of class 12 Chemistry lesson 7 P-Block Elements
most important questions of class 12
most important questions of class 12 Chemistry
most important questions of class 12 Chemistry chapter 7
most important questions of class 12 Chemistry chapter 7 P-Block Elements
most important questions of class 12 Chemistry lesson 7
most important questions of class 12 Chemistry lesson 7 P-Block Elements
mcqs test,
class 12 term 1 exam mcqs,
class 12 Chemistry term 1 exam mcqs,
class 12 Chemistry P-Block Elements term 1 exam mcqs,
class 12 Chemistry chapter 7,
class 12 Chemistry chapter 7 P-Block Elements,
cbse term 1 exam mcqs of class 12 Chemistry P-Block Elements,
objective questions of class 12 Chemistry chapter 7,
mcqs of class 12 Chemistry P-Block Elements,
class 12 Chemistry lesson 7 P-Block Elements mcqs,
mcqs of P-Block Elements 12 Chemistry,